OCEAN OPTICS AND OCEAN FACTS
This chapter deals with ocean optics and some interesting facts about the ocean.
10.1INTRODUCTION TO OPTICAL PROPERTIES OF THE OCEAN
The optical properties of sea water are grouped into inherent and apparent properties. Inherent optical properties (IOPs) are those properties that depend only upon the medium and therefore are independent of the ambient light. The two fundamental IOPs are the absorption coefficient and the volume scattering function. Apparent optical properties (AOPs) are those properties that depend both on the medium (the IOPs) and on the directional structure of the ambient light and display enough regular features and stability to be useful descriptors of a water body. Commonly used AOPs are the irradiance reflectance, the remote sensing reflectance, and various diffuse attenuation functions.
Case 1 waters are those in which the contribution by phytoplankton to the total absorption and scattering is high compared to that by other substances. Absorption by chlorophyll and related pigments therefore plays the dominant role in determining the total absorption in such waters and dissolved organic matter derived from the phytoplankton also contribute to absorption and scattering in case 1 waters. Case 1 water can range from very clear (oligotrophic) to very productive (eutrophic) water, depending on the phytoplankton concentration.
Case 2 waters are everything else, namely, waters where inorganic particles or dissolved organic matter from land drainage contribute significantly to the IOPs, so that absorption by pigments is relatively less important in determining the total absorption. Roughly 98% of the world’s open ocean and coastal waters fall into the case 1 category, but near-shore and estuarine case 2 waters are disproportionately important to human interests such as recreation, fisheries, and military operations.
The constituents are identified operationally based upon how we measure their optical properties and often are grouped by like optical properties. For example, the distinction between particulate and dissolved is operationally defined by the filter type/pore size. It is essential to remember 1) that the strict chemical definition is quite different, 2) filter pore size varies, and 3) keep track of pore sizes to ensure closure (i.e., don’t define dissolved organic matter by the filtrate of a 0.2 micron pore-sized filter and then measure particulates on a 0.7 micron pore size). Similarly, often all the non-phytoplankton particles are lumped into a single compartment as their optical properties are quite similar. Sometimes all the particulate material is lumped together into suspended particulate material (SPM) or part of it into the particulate organic material (POM). This is often done when studying a specific biogeochemical property using optics. The differentiation of dissolved and particulate materials (using a filter) does not imply that the dissolved material is organic, although this is most often the assumption. For example, inorganic dissolved substances such as iron oxides (rust) could contribute in certain cases.
10.1.1Optical Constituents of Seawater
Oceanic waters are a witch’s brew of dissolved and particulate matter whose concentrations and optical properties vary by many orders of magnitude, so that ocean waters vary in color from the deep blue of the open ocean, where sunlight can penetrate to depths of several hundred meters, to yellowish brown in a turbid estuary, where sunlight may penetrate less than a meter. The most important optical constituents of sea water can be briefly described as follows.
1.Sea water (water + inorganic dissolved materials)
2.Phytoplankton
3.Colored (or chromophoric) dissolved organic material (CDOM)
5.Non-phytoplankton organic particles (sometimes referred to as detritus or tripton)
6.Inorganic particles
Sea water: Water itself is highly absorbing at wavelengths below 250 nm and above 700 nm, which limits the wavelength range of interest in optical oceanography to the near ultraviolet to the near infrared. Water contributes to both absorption and scattering by seawater. In clear ocean waters, water effect on ocean color in the visible cannot be neglected and hence must be taken into account. Temperature and salinity affect both absorption and scattering by water and hence must be taken into account when the optical properties of water are computed.
Absorption by water: Absorption as a rich structure due to the excitation of the different vibration modes of the water molecule and water absorption is affected by temperature and salinity.
Elastic scattering by water: Elastic scattering by sea water depends on salinity (~30% increase for range of salinities observed in the oceans), much less so of temperature (~4% between 0 and 26°C) and pressure (~1.3% for an increase in P of 100bar).
Raman scattering by water: In Raman scattering a fraction of the incident light of wave number υο is absorbed and re-emitted at wave number υs = υο − υr. where υr is the Raman shift of a vibration mode of the water molecule. For water υr = 3400 cm−1. Raman scattering is used to calibrate the intensity of the source of a LIDAR system as the signal leaving the ocean is proportional to the intensity of the source.
Phytoplankton: Phytoplanktons have a major effect on the ocean color and are one of the primary reasons for studying it. Additionally, these microscopic, single-cell, free-floating organisms possess chlorophyll, pigment that allows them to harvest the sunlight and through the process of photosynthesis produce energy-rich organic material, while releasing oxygen. That makes them the most important primary producers in the ocean, base of the oceanic food web, and an important component of the global carbon cycle. For all these reasons, it is of a great importance to understand phytoplankton abundance and dynamics.
Absorption by phytoplankton: Phytoplankton absorbs sun light and uses this energy to produce energy rich organic material (photosynthesis). Chlorophylls, present in all phytoplankton cells, will cause two dominant peaks in absorption spectra, primary at blue (440 nm) and a secondary peak in red part of the spectra (675 nm). Presence of other pigments (depending on species) will cause the broadening of blue peak and appearance of additional absorption maxima.
Scattering by phytoplankton: Scattering properties of phytoplankton are important since they are directly related to remote sensing reflectance calculations (via backscattering to absorption ratio). Scattering and backscattering coefficients of phytoplankton as well as the volume scattering function are derived from either theoretical models or direct measurements of the above-mentioned properties. They highly depended on size, shape, and refractive index of all components of the phytoplankton cell. Values of phytoplankton scattering coefficients, when compared to the rest of the oceanic particles, are relatively low, based on their high water content and strong absorptive properties. Exception to the rule are coccolithophores—phytoplankton that produces small calcium carbonate scales, that makes them very effective scatterers and allow us to see coccolithophorid blooms from space.
Fluorescence by phytoplankton: A portion of the light absorbed by phytoplankton cell can be emitted at another, longer wavelength; a process referred to as fluorescence. Several phytoplankton pigments (chlorophylls, pheopigments, and phycobilins) have fluorescence, with chlorophyll a fluorescence being the most significant one. Although fluorescence is only a form of energy dissipation of the absorbed light, secondary to photosynthesis, it is still significant enough to be observed from space. Fluorescence from phytoplankton chlorophyll is often expressed this simplified formula (Falkowski and Kiefer (1985):

Where PAR is the intensity of light impinging
on the cell, [chla] is chlorophyll concentration,  is chlorophyll
specific phytoplankton absorption coefficient, and
Φf is the quantum yield of fluorescence the emission efficiency of the cell.
Phytoplankton fluorescence and intensity depends (via quantum yield
of fluorescence and chlorophyll specific phytoplankton absorption
coefficient) on several factors: taxonomic position of algae,
pigment content and ratios, photo adaptation, physiological state
of phytoplankton, nutrient conditions, and stage of growth.
is chlorophyll
specific phytoplankton absorption coefficient, and
Φf is the quantum yield of fluorescence the emission efficiency of the cell.
Phytoplankton fluorescence and intensity depends (via quantum yield
of fluorescence and chlorophyll specific phytoplankton absorption
coefficient) on several factors: taxonomic position of algae,
pigment content and ratios, photo adaptation, physiological state
of phytoplankton, nutrient conditions, and stage of growth.
Dissolved organic compounds: These compounds are produced during the decay of plant matter. In sufficient concentrations, these compounds can color the water yellowish brown; they are therefore generally called yellow matter or colored dissolved organic matter (CDOM). CDOM absorbs very little in the red, but absorption increases rapidly with decreasing wavelength, and CDOM can be the dominant absorber at the blue end of the spectrum, especially in coastal waters influenced by river runoff.
Colored or chromophoric dissolved organic matter (CDOM): CDOM is an important optical constituent in water often dominating absorption in the blue. It is based on the absorption or fluorescence by material passing through a given filter (most often with pore size of 0.2µm). As such, it is an absorption (or fluorescence) weighted sum of the different dissolved materials in the water. Note that most of the material comprising DOM does not absorb or fluorescence and that there exist inorganic dissolved materials that also absorb (e.g., iron oxides, nitrate) although it is believed that fluorescence is due solely to organic materials. From this discussion, it follows that CDOM is thus not necessarily a good proxy for DOM, particularly in the open ocean.
CDOM absorption: CDOM spectrum is the visible most often described by an exponentially decreasing function:

Where, s is referred to as the
spectral slope and  a reference wavelength. Single bonds, which are most
abundant, will absorb short wavelength radiation while resonance of
multiple bonds, less abundant, absorb longer wavelength radiation.
Since numerically there many more short bonds, the spectrum is
higher at short wavelengths. This explanation is consistent with
the observation that small values of the spectral slope of CDOM,
s are associated with higher
molecular weight materials. For visible wavelength the most common
values of s appear to be near 0.014nm−1,
varying in the visible from 0.007 to 0.026 nm−1.
a reference wavelength. Single bonds, which are most
abundant, will absorb short wavelength radiation while resonance of
multiple bonds, less abundant, absorb longer wavelength radiation.
Since numerically there many more short bonds, the spectrum is
higher at short wavelengths. This explanation is consistent with
the observation that small values of the spectral slope of CDOM,
s are associated with higher
molecular weight materials. For visible wavelength the most common
values of s appear to be near 0.014nm−1,
varying in the visible from 0.007 to 0.026 nm−1.
Elastic scattering by CDOM: CDOM contribution to scattering by seawater is somewhat controversial. By definition, colloids are part of DOM and, if abundant enough, could contribute significantly to scattering (particularly to backscattering) by sea water. However, there is no observational evidence that CDOM contribute significantly to scattering. Thus, currently, CDOM contribution to scattering is most often neglected.
Inelastic scattering by CDOM: One of the primary methods to quantify CDOM is through fluorescence. Since not all dissolve material that absorbs fluorescences, this material is often denoted as FDOM. In general, absorption and fluorescence can vary, but their ratio can vary by orders of magnitude between different locations. The fluorescence of CDOM in the field is often limited to a single excitation/emission band pair. With lab instrumentation, 2-dimensional excitation emission spectra (EEMS) are measured and used to characterize the FDOM based on the size and presence of known excitation emission peaks.
Bubbles: Bubbles in the upper ocean are primarily generated by breaking waves. When wind speed exceeds 7 ms−1, field observations have shown that a stratus layer of bubbles forms under the sea surface and persist through continuous supply of bubbles by frequent wave breaking and the subsequent advection by turbulence. As wind subsides, bubbles that have been injected will evolve under the effects of buoyancy and gas diffusion and merge into the background population on time scales of minutes to hours. When wind speeds are lower than 3 ms−1, few waves break. Once formed, bubbles are coated with surfactant material almost instantaneously and the accumulation of organic films onto their surfaces provides a stabilizing mechanism against surface tension pressure and gas diffusion.
Organic particles: Biogenic particles occur in many forms.
Bacteria: Living bacteria in the size range 0.2–1.0 µm can be significant scatterers and absorbers of light, especially at blue wavelengths and in clean oceanic waters, where the larger phytoplankton is relatively scarce.
Phytoplankton: These ubiquitous microscopic plants occur with incredible diversity of species, size (from less than 1 µm to more than 200 µm), shape, and concentration. Phytoplankton are responsible for determining the optical properties of most oceanic waters. Their chlorophyll and related pigments strongly absorb light in the blue and red and thus, when concentrations are high, determine the spectral absorption of sea water. Phytoplankton are generally much larger than the wavelength of visible light and can scatter light strongly.
Detritus: Nonliving organic particles of various sizes are produced, for example, when phytoplankton die and their cells break apart, and when zooplankton graze on phytoplankton and leave cell fragments and fecal pellets. Detritus can be rapidly photo-oxidized and lose the characteristic absorption spectrum of living phytoplankton, leaving significant absorption only at blue wavelengths. However, detritus can contribute significantly to scattering, especially in the open ocean.
Inorganic particles: Particles created by weathering of terrestrial rocks can enter the water as windblown dust settles on the sea surface, as rivers carry eroded soil to the sea, or as currents resuspend bottom sediments. Such particles range in size from less than 0.1 µm to tens of micrometers and can dominate water optical properties when present in sufficient concentrations. Particulate matter is usually the major determinant of the absorption and scattering properties of sea water and is responsible for most of the temporal and spatial variability in these optical properties. A central goal of research in optical oceanography is to understand how the absorption and scattering properties of these various constituents relate to the particle type (e.g., microbial species or mineral composition), present conditions (e.g., the physiological state of a living microbe, which in turn depends on nutrient supply and ambient lighting), and history (e.g., photo oxidation of pigments in dead cells). Bio-geo-optical models have been developed that attempt (with varying degrees of success) to predict the IOPs in terms of the chlorophyll concentration or other simplified measures of the composition of a water body.
10.1.2Inherent Optical Properties (IOP) Variability
Inherent optical properties (IOPs) depend only on the properties of the medium and its constituents, which include spectral absorption and scattering coefficients—a(λ) and b(λ), respectively, where λ represents wavelength. The fundamental IOPs are the absorption coefficient and the volume scattering function, as various scattering coefficients (e.g., total, backward) can be determined by integration of the volume scattering function over the appropriate angles. An important characteristic of IOPs is that they are additive. This means that, for a seawater sample containing a mixture of constituents, the absorption and scattering coefficients of the various constituents are independent and the total coefficient can be determined by summation. This fact arises from the definition of IOPs with respect to collimated light. The current methods to measure IOPs can only approximate ideal light field and collection geometry, so corrections are sometimes required to obtain adequate estimates of true IOPs. To explain natural variability in total IOPs and to derive estimates of ecologically and bio–geo-chemically relevant constituents from measured IOPs, it is useful to identify categories of constituents, each of which makes a distinct contribution to the total IOPs. Typically, categories are selected on a combination of operational and functional criteria. For total a(λ), for example, contributions from water aw(λ), chromophoric dissolved organic matter (CDOM) aCDOM(λ), phytoplankton aph(λ) and non-algal particles (NAP) aNAP(λ):

Similar summations can be applied to other IOPs such as b(λ), backscattering bb(λ) and the beam attenuation coefficient c(λ), which is defined as the sum

It should be emphasized that (eqn.1) represents an example set of constituent categories, and the concept can be generalized to as many levels as practical or important for specific problems; The following sections focus on water constituents and their effects on IOPs.
Absorption CDOM: For optical oceanographers and many marine chemists, CDOM is operationally defined by its passage through a small pore size filter (usually 0.2 µm) and its ability to absorb visible and ultraviolet radiation. CDOM is a poorly characterized portion of the total dissolved organic matter (DOM) pool and there are no routine analytical techniques for chemically quantifying total CDOM; for this reason, CDOM is frequently quantified in terms of its measurable optical properties (i.e. absorption or fluorescence). Especially in coastal waters with substantial riverine inputs, absorption by CDOM can be very high compared with other constituents, and so it influences the quantity and color of light penetrating into and reflecting from the upper ocean. Even in the open ocean, however, absorption by CDOM cannot be neglected. CDOM absorption is characterized by smoothly varying spectral dependence with amplitude tending to increase exponentially towards blue and ultraviolet wavelengths. For this reason, its contribution to absorption is usually represented by an exponential function:

Where λ0 represents a reference wavelength and S is the slope of the exponential increase with decreasing wavelength. Both the amplitude and the spectral slope of aCDOM depend on the composition of the dissolved organic matter pool and this in turn depends on a variety of source and sinks processes. Terrestrial input, primarily from riverine sources, is significant; in addition, other processes including photo oxidation (enhanced by CDOM’s strong ultraviolet absorption) and local microbial activity can lead to production or loss of different forms of CDOM.
Phytoplankton: As photosynthetic organisms, phytoplankton contain high concentrations of pigments that harvest energy from sunlight. These pigments consist of different chlorophylls and carotenoids present in varying amounts and each type of pigment has spectral properties that lend phytoplankton their characteristically featured absorption spectra. Due to the ubiquitous presence of chlorophylls, absorption peaks at blue (~440 nm) and red wavelengths (~675 nm) are always present, with the blue peak broadened and enhanced by accessory pigments. Pigment compositions, and thus general light absorption characteristics, are partly constrained by phylogeny, but there are also important variations associated with environmental factors that affect growth. Pigment composition and physiological status also affect the amount of light energy absorbed by phytoplankton that is reemitted as fluorescence at red wavelengths. The first order source of variation in light absorption by phytoplankton is total biomass and this, of course, depends on complex ecological and environmental factors that regulate phytoplankton growth and loss rates. Secondary effects with important consequences for how phytoplankton absorb light include variations in intracellular pigment concentration and composition and variations in cell size and shape; size and shape directly impact pigment package effects.
Non-algal particles (NAP): Marine particles besides phytoplankton are also known to absorb light. In natural samples, it is difficult to separate the broad category of NAP into its different contributors, which can include heterotrophic organisms such as bacteria and micrograzers, other organic particles of a detrital nature such as fecal pellets and cell debris, and various mineral particles of both biogenic (e.g., calcite liths and shells) and terrestrial (e.g., clays and sand) origin. In comparison with phytoplankton, much less is known about the optical properties of these particles, but some generalizations have emerged. In coastal and open ocean waters, total NAP absorption tends to exhibit an absorption spectrum that monotonically increases with decreasing wavelength, similar in form to that observed for CDOM. Consequently, aCDOM can be replaced by aNAP in eqn.1 to provide an adequate description of NAP absorption. Because of the similar spectral character of CDOM and NAP, for some applications these pools have been combined into a single class, referred to as colored detrital matter (CDM; which is technically a misnomer as it also includes living and inorganic matter), whose absorption follows eqn.1 with a composite S parameter that will vary with the relative contributions of CDOM and NAP.
Scattering and backscattering: All particulate material whose index of refraction differs from the surrounding medium will scatter light. The amount of scattering is influenced by particle size and shape and by any absorption that occurs within the particle. In contrast to absorption, scattering is not completely characterized simply by specifying its wavelength dependence; it also has angular dependence. Total scattering is summed over all possible scattering angles, but it is also possible to define scattering coefficients over some angular subsets. Backscattering, which is simply scattering integrated over the backward hemisphere with respect to the direction of light incidence, is a quantity that has received a lot of attention due to its importance for ocean color interpretation. As for total scattering, backscattering depends on particle concentration, size, shape, and complex refractive index. Theoretical considerations show that backscattering is generally enhanced relative to forward scattering as particle size decreases, so different particles may dominate total scattering and backscattering in natural waters. For a given wavelength, scattering by polydisperse particles tends to increase with average particle size and with the average real part of the refractive index, so highly refractive mineral particles scatter more light than a population of bacteria of similar concentration and size distribution, for example. Furthermore, the wavelength dependence of the scattering cross section tends to be steeper for smaller particles. Because of its extreme small size (usually defined <0.2 µm) and relatively dilute nature (compared with water molecules, for example), light scattering by CDOM can be neglected.
In general, ocean color remote sensing is a passive remote technique. The sensor, mounted on a satellite, an aircraft or other remote platform, detects the radiometric flux at several selected wavelengths in the visible and near-infrared domains.
The signal received by the sensor is determined by different processes in the water, as well as in the atmosphere [Figure 10.1].
1.Scattering of sunlight by the atmosphere
2.Reflection of direct sunlight at the sea surface
3.Reflection of sunlight at sea surface
4.Light reflected within the water body
Only the portion of the signal originating from the water body contains information on the water constituents; the remaining portion of the signal, which takes up more than 80% of the total signal, has to be assessed precisely to extract the contribution from the water body. There are two strategies to derive oceanic constituents from the signal of ocean color sensor at top of atmosphere (TOA), a one-step method and a two-step method. For the traditionally used two-step method, the water leaving radiance (or reflectance) is firstly derived from the signal at TOA (this procedure is called atmospheric correction), and then oceanic constituents are retrieved from water leaving radiance (or reflectance). For the one-step method, oceanic constituents are directly derived from the signal at TOA. The one-step method assumes that radiative transfer in the ocean and atmosphere is coupled. The oceanic constituents and aerosol properties are simultaneously derived from satellite measurements at TOA by using the entire spectrum available to ocean color instruments.

FIGURE 10.1 Sketch of different origins of light received by space-borne sensor.
10.2RETRIEVAL OF OCEANIC CONSTITUENTS FROM OCEAN COLOR MEASUREMENTS
There are three major issues in the retrieval of oceanic constituents from ocean color:
How to quantify the relationship between optically significant oceanic constituents and inherent optical properties (IOPs)?
How do IOPs determine ocean color?
How to obtain oceanic constituents from ocean color measurements?
The first two issues are the so-called forward problem, and the last issue is the so-called inverse problem.
The forward problem: The forward problem is solved by radiative transfer theory. Radiative transfer theory describes the relationship between the IOPs of the oceanic constituents and the ocean color. Based on radiative transfer theory, two different approaches relating the ocean color to IOPs have been developed: one analytical and one numerical. The most-used analytical expression relates the hemispherical reflectance R just below the sea surface to the absorption coefficient a and back scattering coefficient bb and was

The proportionality factor f varies between approximately 0.3 to 0.5, depending on the ambient light field and the optical properties of water. Another analytical expression relating the remote sensing reflectance to the IOPs of oceanic constituents was derived as

where t is the transmittance of the air-sea interface, Q is the upwelling irradiance-to-radiance ratio, which is a function of the solar zenith angle and optical properties of water, and n is the real part of the refractive index of seawater. The numerical approach is based on simulations of radiative transfer. It allows including all factors determining the ocean color, i.e., IOPs, rough sea surface, observation geometry, inelastic scattering processes, etc., and has a potential for the development of more advanced retrieval methods. Another advantage is to avoid errors due to eventually poor approximation of the factor Q and the parameter f.
The inverse problem: The determination of the oceanic constituents from ocean color is a parameter estimation problem, where a set of parameters C = {ci, i = 1, ..., I} are estimated from a set of measurements R = {rj, j = 1, ..., J}. The functional relationship between measurements and parameters can be expressed as:
R = g(C) (10.3)
Inverting Equation 10.3, one obtains the set of parameters C from the set of measurements R:
C = g−1(R) (10.4)
In this, C represents three different oceanic constituents: pigment, suspended particulate matter and colored dissolved organic matter, while R is either the remote sensing reflectance, defined as the ratio of water leaving radiance to downwelling irradiance or the hemispherical reflectance, defined as the ratio of upwelling to downwelling irradiance, at sea level in J spectral channels.
If g would be a linear function, one could derive the inverse function g−1, and such obtain the oceanic constituents from the measured spectral reflectance. However, the functional relationship between the oceanic constituents and the resulting reflectance is complex and nonlinear. It is therefore mostly impossible to achieve an analytic inversion of g. The traditional way to overcome this problem is to make assumptions on the functional form of g−1 and then to solve Equation 10.4 by regression techniques or other statistical methods. In recent years, artificial neural networks (ANN) have been increasingly applied to remote sensing data from ocean observing instruments, among those scatterometers and ocean color sensors. ANN techniques are well suited for solving nonlinear problems. No assumptions on the functions g or g−1 defined in Equations 10.3 or 10.4 are required. The training of the ANN requires considerable computational effort, its application is very fast. Therefore, ANN techniques are a promising method to derive oceanic constituents from ocean color data.
10.2.1Retrieval of Oceanic Constituents from Ocean Color at TOA
Traditionally, the retrieval of oceanic constituents is performed by a two-step process: atmospheric correction followed by a bio-optical algorithm to obtain the desired parameters. The atmospheric correction algorithms which are commonly used are based on the black pixel assumption. These algorithms were primarily designed for clear, deep ocean areas. The information about atmospheric aerosols is derived from channels in the red and near infrared (above 670 nm), where the water leaving radiance is close to zero. The derived aerosol information is extrapolated towards the visible channels and the atmospheric contribution is calculated and removed for full spectrum. For the turbid coastal environment, the ocean can no longer assumed to be black in the red and near-infrared because of strong back scattering by suspended materials. Under these conditions, the black pixel assumption is no longer valid for deriving information on atmospheric aerosols. Thus, the algorithms developed for applications to clear ocean waters cannot be easily modified to retrieve water leaving radiance from remote sensing data acquired over the coastal environments.
10.2.2The Atmospheric Correction Problem
An instrument views the ocean from a satellite or an aircraft measures upwelling radiances. These include contributions by the atmosphere, the water surface, and the water column. The atmospheric contribution La comes from solar radiance that is scattered one or more times by atmospheric gases and aerosols into the direction of the sensor. The surface reflected radiance (sun and sky glint) Lr is downwelling solar radiance that is reflected toward the sensor by the water surface. The water leaving radiance Lw comes from light that penetrates the ocean, is changed by the absorbing and scattering components in the water, and is then scattered into an upward direction that eventually leaves the sea surface in the sensor direction. Figure 10.2 shows this conceptually.

FIGURE 10.2 Contributions to the total upwelling radiance above the sea surface, Lu. Yellow arrows are the sun’s unscattered beam; orange arrows are atmospheric path radiance La; red is surface-reflected radiance Lr; green is water-leaving radiance Lw. Thick arrows represent single-scattering contributions; thin arrows illustrate multiple scattering contributions.
Radiance reflected by the sea surface contains information about the wave state of the surface, which may be of interest in it or which, for example, may be useful for detection of surface oil slicks. Radiance scattered by the atmosphere along the path between the sea surface and the sensor contains information about atmospheric aerosols and other atmospheric parameters. However, only the water leaving radiance carries information about the water column and bottom conditions. A sensor looking downward measures the total radiance Lu = La + Lr + Lw and cannot separate the various contributions to the total. Atmospheric correction refers to the process of removing the contributions by surface glint and atmospheric scattering from the measured total to obtain the water leaving radiance. The atmospheric correction problem becomes even more intimidating when effects of sun and sensor viewing directions, atmospheric conditions, and surface wave state are considered.
Most remote sensing retrieval algorithms are developed and use the remote sensing reflectance Rrs = Lw / Ed or an equivalent nondimensional reflectance ρ = πLw/Ed. (If Lw were directionally isotropic, Rrs = 1 / π, so is ρ the ratio of the actual Rrs to an idealized isotropic remote sensing reflectance. The π carries units of steradian. The use of an apparent optical property like Rrs or ρ minimizes the effects of external environmental conditions like sun angle on the magnitude of the spectra.
10.3OCEAN OPTICS DIP PROBE SPECTROMETERS
A solution that is colored obtains this color by absorbing light of a complementary color in the visible spectrum. Absorbance refers to the amount of light that is absorbed by the sample. Spectrometers measure the percentage of light transmitted at a particular wavelength. The absorbance is then calculated from the percent transmittance by the relationship:
Abs = (2-log %transmittance)
A spectrophotometer has a source of light that is directed over a specific path. In the Ocean Optics system this path is the entire blue fiber optic cable. The light is produced with a tungsten halogen light source, the blue box in the picture on the right. This light goes into the long blue fiber optic cable to the dip probe seen in Figure 10.3. Fiber-optic cables are widely used in the telecommunications industry because they can transmit light over long distances without intensity losses or signal dispersion. At the open part of the dip probe tip, the light interacts with the sample. The path length of this part of the dip probe is 1 cm in length. Depending on the nature of the molecules in the sample, some of the light will be absorbed. The remaining light is then reflected through the second fiber-optic cable that leads to a detector inside the computer, seen at the right. The detector measures the number of photons of light that reach it and converts this measurement to electrical current. To perform a measurement using a spectrophotometer, the instrument must first be calibrated at 0% and 100% transmittance.

FIGURE 10.3 Fiber optic cable and light source.
At 0% transmittance, all of the light produced by the light source is absorbed and no photons reach the detector. For the ocean optics system, the calibration for 0% transmittance is performed by blocking the light by placing a folded sheet of paper in the slit of the light source. Next the instrument is calibrated for 100% transmittance using a solution called the blank, which contains all sources of absorbance except the analyte of interest. Sometimes the blank solution is a complicated mixture (i.e., urine or motor oil). For other analyses a pure solvent, such as distilled water, is used as the blank. A new blank is measured for every set of analyses. When the dip probe is placed in the blank solution, the percent transmittance is defined to be 100%. Thus when the dip probe is placed in the sample being measured, the analyte of interest is the only thing that contributes to the absorbance.
The lamp of the light source will need to be turned on approximately 15 minutes prior to analysis so that it reaches a constant temperature and the intensity of the light produced is consistent. The software used with the Ocean Optics equipment is called OOIBase32. To verify that the detector is receiving sufficient signal, the intensity of the highest peak should fall between 3,000 and 4,000 counts when the probe is in a blank solution. To check this, make sure scope mode is turned on and the probe is in your blank solution. Your blank solution should contain all sources of absorbance except the analyte of interest. A spectrum should be acquired that looks like the spectrum in Figure 10.4. If the peak intensity is not between 3,000 and 4,000 counts and yet a spectrum appears, adjust the integration time, shorter to reduce the counts or longer to increase the counts so that the peak intensity lies within this range.
Making a measurement: An absorbance measurement of the sample of interest can be made after the dark spectrum and a reference blank spectrum have been stored and the instrument is calibrated. To make the absorbance measurement for a sample, place the dip probe tip in the sample of interest.
1.Make sure no bubbles are present in the tip area
2.Under Spectrum, select Absorbance mode.
3.Under File - Save - Processed. Name the spectrum.
4.Open the saved spectrum by selecting File - Open - Processed

FIGURE 10.4 Spectrum.
When done with the spectrophotometer, turn off the light source and close the software.
10.3.1Spectrometers
Ocean Optics revolutionized the spectrometer field when it introduced the diffraction grating based spectrometer using a charged coupled device (CCD) for light collection. Combined with fiber optic technology, the Ocean Optics equipment was, and continues to be, a powerful and relatively inexpensive research tool. The spectrometers are very compact (about the size of a deck of playing cards) and light weight. These spectrometers utilize USB technology, making them ideal for field use (in fact, many researchers have their spectrometers in underwater housings for work with corals in their natural habitats).
As mentioned, these spectrometers utilize diffraction gratings to split incoming light into its spectral components. This diffracted light falls upon the CCD array and specialized software analyzes and reports spectral characteristics. No single diffraction grating is efficient for broadband analyses (200–1,100 nm). Ocean Optics offers a choice of 14 gratings for the USB4000. Analyses of UV (down to ~220 nm) and visible radiation will require a specific grating, while work with visible wavelengths and near infrared will call for a different grating. At a minimum, one should decide what part of the spectrum is of interest for analysis. Even with the proper grating, the reported spectral quality is not 100% correct. A grating is efficient over a given spectral range (Ocean Optics uses 30% efficiency as the cutoff). This means these spectrometers generally underreport some wavelengths. It is most efficient at 500 nm and underreports other wavelengths, particularly blue and red wavelengths.
Another important consideration is of expected light intensity. Six variously sized apertures (called slits) are available for the USB4000 (5, 10, 25, 50, 100, 200 µ and “none”—the fiber optic size acts as the light regulating mechanism). Low light applications (such as fluorescence measurements) require larger slits (say, 200 µ), while higher light intensity requires a smaller slit. Optical resolution is a function of slit width and holographic grating. An optical resolution of just a fraction of a nanometer is possible. However, the optical resolution will be generally between 2–10 nm. These spectrometers are compatible with USB 1.1 and USB 2.0; use of a serial port is possible with the available 5v power supply.
Fiber optics: It is not absolutely necessary to use fiber optic patch cords if the goal is simply to measure lamp spectra—one can merely remove the protective cover from the input aperture and point the spectrometer at the light source. However, fiber optic cords do offer advantages in that they can be tightly attached to the aperture, thus protecting the internal works of the spectrometer (the thought of a drop of saltwater or debris entering the spec housing is frightening). The fiber optic cords also offer other advantages. While it is apparent that cords are a must for connecting optional accessories, it should be noted that the size (diameter) of the cord is also a critical consideration. Very simply, the larger the diameter of the fiber optic cord, the more light is transmitted. This is useful to know if high light intensity saturates the CCD array and causes the reported intensity to be above the maximum allowed. Use of a smaller diameter cord could attenuate (weaken) the signal, thus allowing measurement. Fibers are available in the following diameters (in µ): 8 (VIS/NIR only, range of 450–1,000 nm), 50, 100, 200, 300, (for use with UV <250 nm) 400, 600, and 1,000. Fibers are usually 2 meters in length, and custom lengths are available.
Software and applications:
SpectraSuite: SpectraSuite is Java-based software that operates with Windows 98/Me/2000/XP, Mac OSX, and Linux w/USB port. Light measurements are usually reported as counts (a generic term) but it is possible to measure absolute irradiance. Graphical data can be copied, and numerical data exported to spreadsheets such as Excel. Integration time is programmable, as are functions such as averaging and boxcar smoothing. Two spectral charts can be open at one time thus allowing simultaneous use of two spectrometers (especially useful when observed fluorescence excitation and emission wavelengths).
OOISensors: Software specifically for use with fluorescent pH and dissolved oxygen probes.
OOIIrrad: Software for measure of relative and absolute irradiance.
10.3.2Ocean Optics Visible Spectrophotometer
A spectrophotometer is an instrument capable of measuring the absorbance or transmittance of a sample at a selected wavelength or range of wavelengths. A tungsten light bulb or other light source in the instrument produces white light. The light is focused through the sample and a diffraction grating then disperses the wavelengths from the lamp’s continuous spectrum. The light that has passed through the sample (i.e., the transmitted light) and been dispersed strikes an array of detectors: one for each wavelength. These detectors record the amount of transmitted light at each wavelength. The signal given by each detector is used to calculate the absorbance at each wavelength; the computer displays the signal as a plot of absorbance versus wavelength. This graph is called the spectrum of the sample. Simple color meters use red, green, and blue filters in combination with diodes or sensor pixels for measurement. More advanced systems use tri-stimulus filters. These work well for incandescent light sources, but are less accurate for LEDs. Handheld color meters may measure up to 20 wavelength bands, but this is not enough for research or high accuracy measurements. To detect small color changes, very high color resolution is necessary. By capturing the complete spectrum, the color measurement made by a spectrometer allows careful and detailed analysis of data. Color meters and analyzers based on filters or detection over specific bands simply leave a lot of information on the table to better inform the color measurement.
Some color analyzers are also strongly dependent on lighting conditions, since objects tend to appear different colors under different illumination. With the right lighting, two objects can appear to be identical in color even if the reflected spectral power distributions differ, an effect called metamerism. If the lighting changes, however, the colors can look significantly different. This makes controlled lighting conditions essential to consistent results. When color measurements are made with a spectrometer, a full reflected or emissive spectrum is the starting point for all calculations. That allows the data to be analyzed in different ways, and even recalculated at a later date to change the observer, the illuminant, or the color space. It offers maximum flexibility with the same high accuracy as if the calculation had been performed that way initially.
10.4INTERESTING FACTS ABOUT THE OCEAN
Area: about 140 million square miles (362 million sq km), or nearly 71% of the Earth’s surface.
Average depth: 12,200 ft (3,720 meters).
Deepest point: 36,198 ft (11,033 m) in the Mariana Trench in the western Pacific.
 If you could evaporate all the
water out of all the oceans and spread the resulting salt over the
Earth, you would have a 500-ft layer covering everything.
If you could evaporate all the
water out of all the oceans and spread the resulting salt over the
Earth, you would have a 500-ft layer covering everything.
 Do you know that life in the
ocean varies as we go deep? Plants grow to a depth of about 107
meters. Fish color changes; fish living near surface are often
blue, green, or violet. In the twilight zone, which is 180 meters
down, fish are silver or light colored. Many fish living 3,000
meters down in the dark ocean waters have their own lights.
Do you know that life in the
ocean varies as we go deep? Plants grow to a depth of about 107
meters. Fish color changes; fish living near surface are often
blue, green, or violet. In the twilight zone, which is 180 meters
down, fish are silver or light colored. Many fish living 3,000
meters down in the dark ocean waters have their own lights.
 The Red Sea in the Indian Ocean
has the saltiest water; it is also known as Dead Sea as its water
is so salty that nothing can remain alive in it.
The Red Sea in the Indian Ocean
has the saltiest water; it is also known as Dead Sea as its water
is so salty that nothing can remain alive in it.
 The Dead Sea is so salty because
it is surrounded by a hot desert whose intense heat causes sea
water to evaporate faster, and thus as much salt remains in the sea
as water goes into air. The Dead Sea exerts a lot of upwards force
due to its large quantity of salt, so people remain afloat or can
swim with no effort.
The Dead Sea is so salty because
it is surrounded by a hot desert whose intense heat causes sea
water to evaporate faster, and thus as much salt remains in the sea
as water goes into air. The Dead Sea exerts a lot of upwards force
due to its large quantity of salt, so people remain afloat or can
swim with no effort.
 The Pacific is the largest and
deepest ocean in the world.
The Pacific is the largest and
deepest ocean in the world.
 The Mercury, Gemini, and Apollo
spacecraft landed in the Atlantic and Pacific oceans when they
returned to earth.
The Mercury, Gemini, and Apollo
spacecraft landed in the Atlantic and Pacific oceans when they
returned to earth.
 The Indian Ocean is the warmest
ocean. The temperature of surface water sometimes touches 36.6
degrees (C).
The Indian Ocean is the warmest
ocean. The temperature of surface water sometimes touches 36.6
degrees (C).
 The scientists who specialize in
study of oceans are called oceanographers.
The scientists who specialize in
study of oceans are called oceanographers.
 “Pacific” means “peaceful.” When Europeans first
found it, they found it very calm and peaceful, so they named it
“Pacific.”
“Pacific” means “peaceful.” When Europeans first
found it, they found it very calm and peaceful, so they named it
“Pacific.”
 An estuary is a place where a
river flows into the sea.
An estuary is a place where a
river flows into the sea.
 Mangroves are trees and shrubs
that grow on sea shores and estuaries. Their roots can breathe in
oxygen.
Mangroves are trees and shrubs
that grow on sea shores and estuaries. Their roots can breathe in
oxygen.
 Splash zones are parts of the
beaches which are covered by water as the tide come.
Splash zones are parts of the
beaches which are covered by water as the tide come.
 Tsunami is a Japanese word
meaning “high sea-waves.”
Tsunami is a Japanese word
meaning “high sea-waves.”
 Buoys are colored metal floats
which are anchored to the sea bed, used to warn sailors about
dangers of rocks, sand banks, wreckage, etc.
Buoys are colored metal floats
which are anchored to the sea bed, used to warn sailors about
dangers of rocks, sand banks, wreckage, etc.
 A knot is a measure of speed at
sea. One knot equals 1.85 kilometers per hour.
A knot is a measure of speed at
sea. One knot equals 1.85 kilometers per hour.
 Scuba diving means diving under
water with the help of scuba equipment for under-water
breathing.
Scuba diving means diving under
water with the help of scuba equipment for under-water
breathing.
 Salt is produced by evaporating
seawater. This is done by flooding salt pans or salt farms with
seawater and allowing evaporation by the Sun to occur. Salt is left
behind by evaporating seawater.
Salt is produced by evaporating
seawater. This is done by flooding salt pans or salt farms with
seawater and allowing evaporation by the Sun to occur. Salt is left
behind by evaporating seawater.
 The world’s oceans contain enough
water to fill a cube with edges over 1,000 kilometers (621 miles)
in length.
The world’s oceans contain enough
water to fill a cube with edges over 1,000 kilometers (621 miles)
in length.
 Ocean tides are caused by the
Earth rotating while the Moon and Sun’s gravitational pull acts on
ocean water.
Ocean tides are caused by the
Earth rotating while the Moon and Sun’s gravitational pull acts on
ocean water.
 While there are hundreds of
thousands of known marine life forms, there are many that are yet
to be discovered. Some scientists suggest that there could be
millions of marine life forms out there.
While there are hundreds of
thousands of known marine life forms, there are many that are yet
to be discovered. Some scientists suggest that there could be
millions of marine life forms out there.
 Oceans are frequently used as a
means of transport, with various companies shipping their products
across oceans from one port to another.
Oceans are frequently used as a
means of transport, with various companies shipping their products
across oceans from one port to another.
 The largest ocean on Earth is the
Pacific Ocean; it covers around 30% of the Earth’s surface.
The largest ocean on Earth is the
Pacific Ocean; it covers around 30% of the Earth’s surface.
 The Pacific Ocean contains around
25,000 different islands, many more than are found in Earth’s other
oceans.
The Pacific Ocean contains around
25,000 different islands, many more than are found in Earth’s other
oceans.
 The Pacific Ocean is surrounded
by the Pacific Ring of Fire, a large number of active
volcanoes.
The Pacific Ocean is surrounded
by the Pacific Ring of Fire, a large number of active
volcanoes.
 The second-largest ocean on Earth is the Atlantic
Ocean; it covers over 21% of the Earth’s surface.
The second-largest ocean on Earth is the Atlantic
Ocean; it covers over 21% of the Earth’s surface.
 The Atlantic Ocean’s name refers
to Atlas of Greek mythology.
The Atlantic Ocean’s name refers
to Atlas of Greek mythology.
 The Bermuda Triangle is in the
Atlantic Ocean.
The Bermuda Triangle is in the
Atlantic Ocean.
 The third-largest ocean on Earth
is the Indian Ocean; it covers around 14% of the Earth’s
surface.
The third-largest ocean on Earth
is the Indian Ocean; it covers around 14% of the Earth’s
surface.
 During winter the Arctic Ocean is
almost completely covered in sea ice.
During winter the Arctic Ocean is
almost completely covered in sea ice.
 While some disagree on whether it
is an ocean or just part of larger oceans, the Southern Ocean
includes the area of water that encircles Antarctica.
While some disagree on whether it
is an ocean or just part of larger oceans, the Southern Ocean
includes the area of water that encircles Antarctica.
 World Oceans Day is June 8.
World Oceans Day is June 8.
 More than 97% of all our planet’s
water is contained in the ocean.
More than 97% of all our planet’s
water is contained in the ocean.
 The top 10 ft of the ocean hold
as much heat as our entire atmosphere.
The top 10 ft of the ocean hold
as much heat as our entire atmosphere.
 The average depth of the ocean is
more than 2.5 miles.
The average depth of the ocean is
more than 2.5 miles.
 The oceans provide 99% of the
Earth’s living space—the largest space in our universe known to be
inhabited by living organisms.
The oceans provide 99% of the
Earth’s living space—the largest space in our universe known to be
inhabited by living organisms.
 More than 90% of this habitat
exists in the deep sea known as the abyss.
More than 90% of this habitat
exists in the deep sea known as the abyss.
 Less than 10% of this living
space has been explored by humans.
Less than 10% of this living
space has been explored by humans.
 Mount Everest (the highest point
on the Earth’s surface at 5.49 miles) is more than 1 mile shorter
than the Challenger Deep (the deepest point in the ocean at 6.86
miles).
Mount Everest (the highest point
on the Earth’s surface at 5.49 miles) is more than 1 mile shorter
than the Challenger Deep (the deepest point in the ocean at 6.86
miles).
 The longest continuous mountain
chain known to exist in the universe resides in the Atlantic Ocean
at more than 40,000 miles long.
The longest continuous mountain
chain known to exist in the universe resides in the Atlantic Ocean
at more than 40,000 miles long.
 The Monterey Bay Submarine Canyon
is deeper and larger in volume than the Grand Canyon.
The Monterey Bay Submarine Canyon
is deeper and larger in volume than the Grand Canyon.
 The average temperature of the
oceans is 2ºC, about 39ºF.
The average temperature of the
oceans is 2ºC, about 39ºF.
 Water pressure at the deepest
point in the ocean is more than 8 tons per square inch, the
equivalent of one person trying to hold 50 jumbo jets.
Water pressure at the deepest
point in the ocean is more than 8 tons per square inch, the
equivalent of one person trying to hold 50 jumbo jets.
 The Gulf Stream off the Atlantic
seaboard of the United States flows at a rate nearly 300 times
faster than the typical flow of the Amazon River, the world’s
largest river.
The Gulf Stream off the Atlantic
seaboard of the United States flows at a rate nearly 300 times
faster than the typical flow of the Amazon River, the world’s
largest river.
 The world’s oceans contain nearly
20 million tons of gold.
The world’s oceans contain nearly
20 million tons of gold.
 The color blue is least absorbed by seawater; the
same shade of blue is most absorbed by microscopic plants, called
phytoplankton, drifting in seawater.
The color blue is least absorbed by seawater; the
same shade of blue is most absorbed by microscopic plants, called
phytoplankton, drifting in seawater.
 A new form of life, based on
chemical energy rather than light energy, resides in deep-sea
hydrothermal vents along mid-ocean ridges.
A new form of life, based on
chemical energy rather than light energy, resides in deep-sea
hydrothermal vents along mid-ocean ridges.
 A swallow of seawater may contain
millions of bacterial cells, hundreds of thousands of
phytoplankton, and tens of thousands of zooplankton.
A swallow of seawater may contain
millions of bacterial cells, hundreds of thousands of
phytoplankton, and tens of thousands of zooplankton.
 The blue whale, the largest
animal on our planet ever (exceeding the size of the greatest
dinosaurs) still lives in the ocean.
The blue whale, the largest
animal on our planet ever (exceeding the size of the greatest
dinosaurs) still lives in the ocean.
 The gray whale migrates more than
10,000 miles each year, the longest migration of any mammal.
The gray whale migrates more than
10,000 miles each year, the longest migration of any mammal.
 The Great Barrier Reef, measuring
1,243 miles, is the largest living structure on Earth. It can be
seen from the Moon.
The Great Barrier Reef, measuring
1,243 miles, is the largest living structure on Earth. It can be
seen from the Moon.
 More than 90% of the trade
between countries is carried by ships and about half the
communications between nations use underwater cables.
More than 90% of the trade
between countries is carried by ships and about half the
communications between nations use underwater cables.
 More oil reaches the oceans each
year from leaking automobiles and other non-point sources.
More oil reaches the oceans each
year from leaking automobiles and other non-point sources.
 Fish supply the greatest
percentage of the world’s protein consumed by humans.
Fish supply the greatest
percentage of the world’s protein consumed by humans.
 Most of the world’s major
fisheries are being fished at levels above their maximum
sustainable yield; some regions are severely overfished.
Most of the world’s major
fisheries are being fished at levels above their maximum
sustainable yield; some regions are severely overfished.
 The Grand Banks, the pride of New
England fishing for centuries, are closed due to overfishing.
The Grand Banks, the pride of New
England fishing for centuries, are closed due to overfishing.
 Eighty percent of all pollution
in seas and oceans comes from land-based activities.
Eighty percent of all pollution
in seas and oceans comes from land-based activities.
 Three-quarters of the world’s
mega-cities are by the sea.
Three-quarters of the world’s
mega-cities are by the sea.
 By 2010, 80% of people will live
within 60 miles of the coast.
By 2010, 80% of people will live
within 60 miles of the coast.
 Plastic waste kills up to 1
million sea birds, 100,000 sea mammals, and countless fish each
year. Plastic remains in our ecosystem for years harming thousands
of sea creatures every day.
Plastic waste kills up to 1
million sea birds, 100,000 sea mammals, and countless fish each
year. Plastic remains in our ecosystem for years harming thousands
of sea creatures every day.
 Over the past decade, an average
of 600,000 barrels of oil a year has been accidentally spilled from
ships.
Over the past decade, an average
of 600,000 barrels of oil a year has been accidentally spilled from
ships.
 Tropical coral reefs border the shores of 109
countries, most of which are among the world’s least developed.
Significant reef degradation has occurred in 93 countries.
Tropical coral reefs border the shores of 109
countries, most of which are among the world’s least developed.
Significant reef degradation has occurred in 93 countries.
 Although coral reefs comprise
less than 0.5% of the ocean floor, it is estimated that more than
90% of marine species are directly or indirectly dependent on
them.
Although coral reefs comprise
less than 0.5% of the ocean floor, it is estimated that more than
90% of marine species are directly or indirectly dependent on
them.
 There are about 4,000 coral reef
fish species worldwide, accounting for approximately a quarter of
all marine fish species.
There are about 4,000 coral reef
fish species worldwide, accounting for approximately a quarter of
all marine fish species.
 Nearly 60% of the world’s
remaining reefs are at significant risk of being lost in the next
three decades.
Nearly 60% of the world’s
remaining reefs are at significant risk of being lost in the next
three decades.
 The major causes of coral reef
decline are coastal development, sedimentation, destructive fishing
practices, pollution, tourism, and global warming.
The major causes of coral reef
decline are coastal development, sedimentation, destructive fishing
practices, pollution, tourism, and global warming.
 Less than 0.5% of marine habitats
are protected, compared with 11.5% of global land area.
Less than 0.5% of marine habitats
are protected, compared with 11.5% of global land area.
 The High Seas—areas of the ocean
beyond national jurisdiction—cover almost 50% of the Earth’s
surface. They are the least protected part of the world.
The High Seas—areas of the ocean
beyond national jurisdiction—cover almost 50% of the Earth’s
surface. They are the least protected part of the world.
 Although there are some treaties
that protect ocean-going species such as whales, as well as some
fisheries agreements, there are no protected areas in the High
Seas.
Although there are some treaties
that protect ocean-going species such as whales, as well as some
fisheries agreements, there are no protected areas in the High
Seas.
 Studies show that protecting
critical marine habitats—such as warm-and cold-water coral reefs,
seagrass beds, and mangroves—can dramatically increase fish size
and quantity.
Studies show that protecting
critical marine habitats—such as warm-and cold-water coral reefs,
seagrass beds, and mangroves—can dramatically increase fish size
and quantity.
 More than 3.5 billion people
depend on the ocean for their primary source of food. In 20 years,
this number could double to 7 billion.
More than 3.5 billion people
depend on the ocean for their primary source of food. In 20 years,
this number could double to 7 billion.
 Populations of commercially
attractive large fish, such as tuna, cod, swordfish, and marlin
have declined by as much as 90% in the past century.
Populations of commercially
attractive large fish, such as tuna, cod, swordfish, and marlin
have declined by as much as 90% in the past century.
 Each year, illegal longline
fishing, which involves lines up to 80 miles long, with thousands
of baited hooks, kills over 300,000 seabirds, including 100,000
albatrosses.
Each year, illegal longline
fishing, which involves lines up to 80 miles long, with thousands
of baited hooks, kills over 300,000 seabirds, including 100,000
albatrosses.
 As many as 100 million sharks are
killed each year for their meat and fins, which are used for shark
fin soup. Hunters typically catch the sharks,
de-fin them while alive, and throw them back into the ocean, where
they either drown or bleed to death.
As many as 100 million sharks are
killed each year for their meat and fins, which are used for shark
fin soup. Hunters typically catch the sharks,
de-fin them while alive, and throw them back into the ocean, where
they either drown or bleed to death.
 Global by-catch—unintended
destruction caused by nonselective fishing gear, such as trawl
nets, longlines, and gillnets—amounts to 20 million tons a
year.
Global by-catch—unintended
destruction caused by nonselective fishing gear, such as trawl
nets, longlines, and gillnets—amounts to 20 million tons a
year.
 The annual global by-catch
mortality of small whales, dolphins, and porpoises alone is
estimated to be more than 300,000 individuals.
The annual global by-catch
mortality of small whales, dolphins, and porpoises alone is
estimated to be more than 300,000 individuals.
 Ninety-four percent of life on
Earth is aquatic. That makes us land-dwellers a very small
minority.
Ninety-four percent of life on
Earth is aquatic. That makes us land-dwellers a very small
minority.
 About 70% of the planet is ocean,
with an average depth of more than 12,400 feet. Given that photons
(light) can’t penetrate more than 330 feet below the water’s
surface, most of our planet is in a perpetual state of
darkness.
About 70% of the planet is ocean,
with an average depth of more than 12,400 feet. Given that photons
(light) can’t penetrate more than 330 feet below the water’s
surface, most of our planet is in a perpetual state of
darkness.
 Because the architecture and
chemistry of coral is so like human bone, coral has been used to
replace bone grafts in helping human bone to heal quickly and
cleanly.
Because the architecture and
chemistry of coral is so like human bone, coral has been used to
replace bone grafts in helping human bone to heal quickly and
cleanly.
 The deep sea is the largest
museum on Earth: There are more artifacts and remnants of history
in the ocean than in all the world’s museums, combined.
The deep sea is the largest
museum on Earth: There are more artifacts and remnants of history
in the ocean than in all the world’s museums, combined.
 We have only explored less than
5% of the Earth’s oceans. In fact, we have better maps of Mars than
we do of the ocean floor.
We have only explored less than
5% of the Earth’s oceans. In fact, we have better maps of Mars than
we do of the ocean floor.
 The longest mountain range in the
world is under water. Called the Mid-Oceanic Ridge, this chain of
mountains runs through the middle of the Atlantic Ocean and into
the Indian and Pacific oceans. It is more than 35,000 miles long,
has peaks higher than those in the Alps and comprises 23% of the
Earth’s total surface.
The longest mountain range in the
world is under water. Called the Mid-Oceanic Ridge, this chain of
mountains runs through the middle of the Atlantic Ocean and into
the Indian and Pacific oceans. It is more than 35,000 miles long,
has peaks higher than those in the Alps and comprises 23% of the
Earth’s total surface.
 We didn’t send divers down to
explore the Mid-Ocean Ridge until 1973—four years after Neil
Armstrong and Buzz Aldrin walked on the moon—when a French-American
crew of seven entered the 9,000-foot-deep Great Rift in the French
submersible Archimede.
We didn’t send divers down to
explore the Mid-Ocean Ridge until 1973—four years after Neil
Armstrong and Buzz Aldrin walked on the moon—when a French-American
crew of seven entered the 9,000-foot-deep Great Rift in the French
submersible Archimede.
 The ocean boasts an array of
unusual geographic features, such as pillars that reach several
stories high and chimneys that send up sulphuric acid. In the
ocean-floor neighborhood of the Gulf of Mexico, brine pools mark
the floor, along with underwater volcanoes that spew mud and
methane, rather than lava.
The ocean boasts an array of
unusual geographic features, such as pillars that reach several
stories high and chimneys that send up sulphuric acid. In the
ocean-floor neighborhood of the Gulf of Mexico, brine pools mark
the floor, along with underwater volcanoes that spew mud and
methane, rather than lava.
 These wonderful formations aren’t barren. Underwater
hot springs that shoot water that’s 650°F—hot enough to melt
lead—boast a profusion of life, from 10-ft tall tubeworms to giant
clams that function without digestive systems.
These wonderful formations aren’t barren. Underwater
hot springs that shoot water that’s 650°F—hot enough to melt
lead—boast a profusion of life, from 10-ft tall tubeworms to giant
clams that function without digestive systems.
 The part of the ocean farthest
from land lies in the South Pacific and is known as Point Nemo or
“The Pole of Inaccessibility.”
The part of the ocean farthest
from land lies in the South Pacific and is known as Point Nemo or
“The Pole of Inaccessibility.”
The Bermuda Triangle
 Located in the Atlantic Ocean,
the Bermuda Triangle falls between Bermuda, Puerto Rico, and
Florida as shown in above figure.
Located in the Atlantic Ocean,
the Bermuda Triangle falls between Bermuda, Puerto Rico, and
Florida as shown in above figure.
 The Bermuda Triangle has long
been believed to be the site of a number of mysterious plane and
boat incidents have occurred.
The Bermuda Triangle has long
been believed to be the site of a number of mysterious plane and
boat incidents have occurred.
 While it has become part of
popular culture to link the Bermuda Triangle to paranormal
activity, most investigations indicate bad weather and human error
are the more likely culprits.
While it has become part of
popular culture to link the Bermuda Triangle to paranormal
activity, most investigations indicate bad weather and human error
are the more likely culprits.

 Research has suggested that many
original reports of strange incidents in the Bermuda Triangle were
exaggerated and that the actual number of incidents in the area is
similar to that in other parts of the ocean.
Research has suggested that many
original reports of strange incidents in the Bermuda Triangle were
exaggerated and that the actual number of incidents in the area is
similar to that in other parts of the ocean.
 While its reputation may scare
some people, the Bermuda Triangle is part of a regularly sailed
shipping lane, with cruise ships and other boats also frequently
sailing through the area.
While its reputation may scare
some people, the Bermuda Triangle is part of a regularly sailed
shipping lane, with cruise ships and other boats also frequently
sailing through the area.
 Aircraft are also common in the
Bermuda Triangle, with both private and commercial planes commonly
flying through the airspace.
Aircraft are also common in the
Bermuda Triangle, with both private and commercial planes commonly
flying through the airspace.
 Stories of unexplained
disappearances in the Bermuda Triangle started to reach public
awareness around 1950 and have been consistently reported since
then.
Stories of unexplained
disappearances in the Bermuda Triangle started to reach public
awareness around 1950 and have been consistently reported since
then.
 Unverified supernatural
explanations for Bermuda Triangle incidents have included
references to UFO’s and even the mythical lost continent of
Atlantis.
Unverified supernatural
explanations for Bermuda Triangle incidents have included
references to UFO’s and even the mythical lost continent of
Atlantis.
 Other explanations have included magnetic anomalies,
pirates, deliberate sinkings, hurricanes, gas deposits, rough
weather, huge waves, and human error.
Other explanations have included magnetic anomalies,
pirates, deliberate sinkings, hurricanes, gas deposits, rough
weather, huge waves, and human error.
 Some famous reported incidents
involving the Bermuda Triangle include the USS Cyclops and
its crew of 309 that went missing after leaving Barbados in 1918;
the TBM Avenger bombers that went missing in 1945 during a training
flight over the Atlantic; a Douglas DC-3 aircraft containing 32
people that went missing in 1958, with no trace of the aircraft
ever found; and a yacht found in 1955 that had survived three
hurricanes but was missing all its crew.
Some famous reported incidents
involving the Bermuda Triangle include the USS Cyclops and
its crew of 309 that went missing after leaving Barbados in 1918;
the TBM Avenger bombers that went missing in 1945 during a training
flight over the Atlantic; a Douglas DC-3 aircraft containing 32
people that went missing in 1958, with no trace of the aircraft
ever found; and a yacht found in 1955 that had survived three
hurricanes but was missing all its crew.
Coastlines: The total length of the world’s coastlines is about 315,000 miles, enough to circle the equator 12 times. As coastal zones become more and more crowded, the quality of coastal water will suffer, wildlife will be displaced, and the shorelines will erode. Sixty percent of the Pacific and 35% of the Atlantic Coast shoreline are eroding at a rate of a meter every year. More than half the world’s population live within 100 km or 60 miles of the coast. This is more than 2.7 billion people. Rapid urbanization will lead to more coastal mega-cities containing 10 million or more people. By the end of the millennium, 13 out of 15 of the world’s largest cities will be located on or near the coast. Growing population in coastal areas leads to more marine pollution and distribution of coastal habitats. Some 6.5 million tons (6,500,000,000 kilo) of litter finds its way into the sea each year.
Fisheries: The sea provides the biggest source of wild or domestic protein in the world. Each year some 70 to 75 million tons of fish are caught in the ocean. Of this amount, around 29 million tons is for human consumption. Global fish production exceeds that of cattle, sheep, poultry, or eggs. Fish can be produced in two ways: by capture and by aquaculture. Total production has grown 34% over the last decade. The largest numbers of fish are in the Southern Hemisphere due to the fact that these waters are not largely exploited by man. Fifteen out of seventeen of the world’s largest fisheries are so heavily exploited that reproduction can’t keep up, with the result that many fish populations are decreasing rapidly. Species of fish endangered by overfishing are: tuna, salmon, haddock, halibut, and cod. In the 19th century, codfish weighing up to 200 pounds used to be caught. Nowadays, a 40-pound cod is considered a giant. Reason: overfishing.
Rising sea level: The sea level has risen an average of 4–10 inches (10–25 cm) over the past 100 years and scientists expect this rate to increase. Sea levels will continue rising even if the climate has stabilized, because the ocean reacts slowly to changes. Ten thousand years ago the ocean level was about 330 ft (110 meters) lower than it is now. If the entire world’s ice melted, the oceans would rise 200 ft (66 meters).
Volcanic activity: Ninety percent of all volcanic activity on Earth occurs in the ocean. The largest known concentration of active volcanoes (approximately 1,133) on the sea floor is in the South Pacific.
Density: The density of ocean water varies. It becomes denser as it becomes colder, right down to its freezing point of -1.9°C. (This is unlike fresh water, which is most dense at 4°C, well above its freezing point.)
Water temperature: Under the enormous pressures of the deep ocean, seawater can reach very high temperatures without boiling. A water temperature of 400°C has been measured at one hydrothermal vent. The average temperature of all ocean water is about 3.5°C. Almost all deep ocean temperatures are only a little warmer than freezing (39°F).
Ice: Antarctica has as much ice as the Atlantic Ocean has water. Ten percent of the earth’s surface is covered with ice. The Arctic Ocean is the smallest ocean, holding only 1% of the Earth’s seawater. This is still more than 25 times as much water as all rivers and freshwater lakes. The average thickness of the Arctic ice sheet is about 9–10 ft, although there are some areas as thick as 65 ft. In the unlikely event that all the polar ice was to melt, the sea level all over the world would rise 500–600 ft. As a result, 85–90% of the Earth’s surface would be covered with water as compared to the current 71%. The United States would be split by the Mississippi Sea, which would connect the Great Lakes with the Gulf of Mexico. The Arctic produces 10,000 to 50,000 icebergs annually. The amount produced in the Antarctic regions is inestimable. Icebergs normally have a four-year lifespan; they begin entering shipping lanes after about three years.
Carbon dioxide absorption: Oceans absorb between 30% and 50% of the carbon dioxide produced by burning fossil fuel. Carbon dioxide is transported downwards by plankton. Any change in the temperature of the ocean water, influences the ability of plankton to take up carbon dioxide. This has consequences for the ecosystem, because plankton forms the base of the food web.
Reefs: Over 60% of the world’s coral reefs are threatened by pollution, sedimentation, and bleaching due to rising water temperatures caused by global warming. Global Coral Monitoring Network (GCRMN) states that currently 27% of all coral reef worldwide has disappeared and by 2050 only 30% will be left.
Rubbish/contamination: In one year, three times as much rubbish is dumped into the world’s oceans as the weight of fish caught. A single quart of motor oil can contaminate up to 2 million gallons of drinking water.
Oil: Oil is one of the ocean’s greatest resources. It gives us heat for our homes, endless consumer products, and the ability to run the engines of cars, planes, and boats for auto transport all over the world. Nearly one-third of the world’s oil comes from offshore fields in our oceans which, as we’ve seen, can have devastating effects on our ocean’s ecosystems. The transport of ocean oil from the Arabian Gulf, the North Sea, and the Gulf of Mexico reaches all corners of the globe daily. Oil was also born from the sea. Millions of years ago, countless marine microscopic plants (phytoplankton) and animals (zooplankton) lived in the ancient seas as they do today. As they died, the skeletal remains of these tiny organisms settled to the sea floor, mixed with mud and silt, and over millions of years, formed organic-rich sedimentary layers. Other sediments continued to be deposited and further buried the organic-rich sediment layer to depths of thousands of feet, compressing the layers into a rock that would become the source for oil. Over the years, as the depth of the burial increased, pressure increased, along with the temperature. Under such conditions, and over long periods of time, the original skeletal remains of phytoplankton and zooplankton changed, breaking down into simpler substances called hydrocarbons—compounds of hydrogen and carbon. This process continues, although it will be millions of years before the next batch of oil is done cooking. Refined oil is also responsible for polluting the ocean.
Salinity: Some scientists estimate that the oceans contain as much as 50 quadrillion tons (50 million billion tons = 50,000,000,000,000,000) of dissolved solids. If the salt in the ocean could be removed and spread evenly over the Earth’s land surface, it would form a layer more than 500 ft (166 meters) thick, about the height of a 40-story office building. The ocean’s principal dissolved solids are sodium salts (sodium chloride or common salt), calcium salts (calcium carbonate or lime, and calcium sulfate), potassium salts (potassium sulfate), and magnesium salts (magnesium chloride, magnesium sulfate, and magnesium bromide). Atlantic sea water is heavier than Pacific sea water due to its higher salt content. The freezing point of sea water depends on its salt content. Typical ocean water has about 35 grams of salt per liter and freezes at −19°C.
Seawater’s inorganic salt components:
|
Chloride Sodium Sulfate Magnesium Calcium Potassium Carbonic Acid Bromine Boric Acid Strontium Total |
Cl- NA+ SO4-- Mg++ Ca++ K+ HCO3- Br- H3Bo3 Sr++ |
55.04% 30.61% 7.68% 3.69% 1.16% 1.16% 0.41% 0.19% 0.07% 0.04% 99. |
Desalination: Arabian Gulf reverse osmosis plants treat 500,000,000 gallons of sea water to obtain 100,000,000 gallons of fresh water. Daily over 500,000,000 gallons of seawater must be heated to extremely high temperatures. Mixed with toxic chemicals, the seawater is injected under high pressure through a series of membrane filters. Only 100,000,000 gallons of fresh water is generated. The 5:1 ratio of this highly inefficient process means 400,000,000 gallons of untreated water are returned to the sea each day. The higher temperature of the discharged water causes environmental problems. Worse, the super-heated brine discharge has significantly higher levels of total dissolved solids and toxic chemicals are mixed in with it. This pollution is usually discharged back into the sea.
The 10 largest territorial powers (in million sq km):
An estimated 50–80% of all life on earth is found under the ocean surface and the oceans contain 99% of the living space on the planet. Eighty-five percent of the area and 90% of the volume constitute the dark, cold environment we call the deep sea. The average depth of the ocean is 3,795 meters. The average height of the land is 840 meters. The oceans contain 97% of the Earth’s water. Less than 1% is fresh water, and 2–3% is contained in glaciers and ice caps (and is decreasing).
The speed of sound in water is 1,435m/sec—nearly five times faster than the speed of sound in air. The highest tides in the world are at the Bay of Fundy, which separates New Brunswick from Nova Scotia. At some times of the year the difference between high and low tide is 16.3 meters, taller than a three-story building. Earth’s longest mountain range is the Mid-Ocean Ridge more than 50,000km in length, which winds around the globe from the Arctic Ocean to the Atlantic, skirting Africa, Asia, and Australia, and crossing the Pacific to the west coast of North America. It is four times longer than the Andes, Rockies, and Himalayas combined. The pressure at the deepest point in the ocean is more than 11,318 tons/sq meter.
The largest recorded tsunami measured 60 meters above sea level, caused by an 8.9 magnitude earthquake in the Gulf of Alaska in 1899 traveling at hundreds of km/hr.
The average depth of the Atlantic Ocean, with its adjacent seas, is 3,332 meters; without them it is 3,926 meters. The greatest depth, 8,381 meters, is in the Puerto Rico Trench. The Pacific Ocean, the world’s largest water body, occupies a third of the Earth’s surface. The Pacific contains about 25,000 islands (more than the total number in the rest of the world’s ocean combined), almost all of which are found south of the equator. The Pacific covers an area of 179.7 million sq km. The Kuroshio Current, off the shores of Japan, is the largest current. It can travel between 40–121 km/day at 1.6–4.8 km/hr, and extends some 1,006 meters deep. The Gulf Stream is close to this current’s speed. The Gulf Stream is a well-known current of warm water in the Atlantic Ocean. At a speed of 97km/day, the Gulf Stream moves a hundred times as much water as all the rivers on earth and flows at a rate 300 times faster than Amazon, which is the world’s largest river.
Earth’s oceans are unique in the universe—as far as we know: Earth is the only known planet or moon to have large bodies of liquid water on its surface. Our planet lies in the “Goldilocks” zone—not too hot, not too cold, and with enough atmospheric pressure to prevent liquid surface water from evaporating into space. Although we don’t yet know of any other planets or moons with liquid water oceans, it’s likely that they do exist and we just haven’t found them. In our own solar system, there is growing evidence that the planet Mars may have liquid water not on the surface but underground. There is also strong evidence that liquid oceans may be hidden beneath the thick icy surfaces of three of Jupiter’s moons (Europa, Callisto, and Ganymede) and two of Saturn’s moons (Titan and Enceladus).
For every species of marine life we know of, at least another three are yet to be discovered: Our oceans teem with life ranging from the blue whale—the biggest animal on Earth—to tiny microbes. But nobody knows exactly how many different species live in this environment. There is no data for around 20% of the ocean’s volume. The Census of Marine Life, a 10-year international project to identify life in our oceans, found nearly 250,000 species. But scientists believe a least a million species of marine life could be out there, and that’s not counting the tens or even hundreds of millions of kinds of microbes that make up the majority of marine life. What we do know is that ocean life survives in the most extreme environments. Scientists have found life that can survive in temperatures that melt lead, where seawater freezes into ice, or there’s no light or oxygen. In fact, the dark ocean zone between 1,000 and 5,000 meters known as the abyssal zone has a far greater range of marine life than we once thought.
Water takes around 1,000 years to travel all the way around the whole globe: The oceans not only have waves, tides, and surface currents—they also have a constantly moving system of deep-ocean circulation driven by temperature and salinity. Known as the global ocean conveyor belt or thermohaline current (thermo = temperature, haline = salinity), this deep ocean current gets one of its starts in the polar region near Norway. As sea ice forms, the water left behind becomes saltier and denser and begins to sink, making room for warmer and less dense incoming surface water, which in turn eventually becomes cold and salty enough to sink. The cold dense water flows along the ocean bottom all the way from the Northern Hemisphere to the Southern Ocean, where it merges with more cold dense water from Antarctica and is swept into the Indian and Pacific Oceans as shown in below figure. Eventually it mixes with warmer water and rises to the surface before finding its way back to the Atlantic. It can take 1,000 years to complete this cycle.
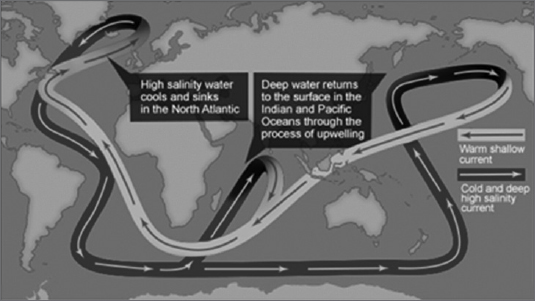
Half of all the oxygen we breathe is produced in the ocean: Some of this oxygen is produced by sea weeds and sea grasses, but the vast majority of the oxygen is produced by phytoplankton, microscopic single-celled organisms that have the ability to photosynthesize. These tiny creatures live in the surface layer of the ocean (and in lakes and rivers) and form the very base of the aquatic food chain. During photosynthesis, phytoplankton removes carbon dioxide from sea water and release oxygen. The carbon becomes part of their bodies.
Oceans hold around 50 times more carbon than the atmosphere
Cold water can dissolve much more CO2 than warm water, so the cold polar regions are net absorbers of CO2. But as the cold water finds its way to warmer tropical areas, the oceans release CO2 back into the atmosphere. The equatorial Pacific is thought to be the biggest single natural source of CO2 in the atmosphere. Most of this carbon is exchanged with the atmosphere on a timescale of several hundred years.

Prior to the industrial revolution, the uptake and release of CO2 on land and ocean was in a dynamic equilibrium. Since then, the oceans are thought to have absorbed about half of the carbon dioxide released from the burning of fossil fuels, with the rest remaining in the atmosphere.
EXERCISES: PART A (ANSWER IN A WORD OR A SENTENCE)
1.What are the two different types of optical properties of sea water?
2.What are the wavelengths that light absorbs highly?
3.What is Raman scattering by water?
4.__________ is an instrument capable of measuring the absorbance or transmittance of a sample at a selected wavelength.
5.What is the software used to measure relative and absolute irradiance?
6.__________ software is used with fluorescent pH and dissolved oxygen probes.
EXERCISES: PART B (ANSWER IN A PAGE)
1.What is important about case 2 waters?
2.Write the properties of case 1 water.
3.What are inherent optical properties?
4.What is meant by apparent optical properties?
5.Write about the light effect on non-algal particles.
6.What is scattering and backscattering?
7.Describe the method to solve forward problem.
8.Write about the atmospheric correction problem.
9.Write a note on spectrometers.
10.Write about the ocean optics visible spectrophotometer.
EXERCISES: PART C (ANSWER WITHIN 2 OR 3 PAGES)
1.Write the optical constituents of sea water.
2.Explain the absorption, scattering, and fluorescence by phytoplankton.
3.What are the effects of optic properties on CDOM?
4.In detail, write about the retrieval of oceanic constituents from ocean color measurements at sea level.
5.Describe the operation of ocean optics dip probe spectrometers.
WEB LINKS
