Probably all of us are familiar with
the guessing game ‘animal, vegetable or mineral’. The implicit
assumption in the name of this game is that plants and animals are
completely different from one another. True, they are both living
organisms, but that’s where we feel the similarity ends. We may be
able to get on board with the idea that somewhere back in the murky
evolutionary past, humans and microscopic worms have a shared
ancestor. But how often do we ever wonder about the biological
heritage we share with plants? When do we ever think of carnations
as our cousins?
Yet animals and plants are surprisingly
similar in many ways. This is especially the case when we consider
the most advanced of our green relatives, the flowering plants.
These include the grasses and cereals that we rely on for so much
of our basic food intake, and the broad-leaved plants, from
cabbages to oak trees and from rhododendrons to cress.
Animals and the flowering plants are each
made up of lots of cells; they are multicellular organisms. Many of
these cells are specialised for particular functions. In the
flowering plants these include cells that transport water or sugars
around the plant, the photosynthesising cells of the leaves and the
food storing cells of the roots. Like animals, plants have
specialised cells which are responsible for sexual reproduction.
The sperm nuclei are carried in pollen and fertilise a large egg
cell, which ultimately gives rise to a zygote and a new individual
plant.
The similarities between plants and
animals are more fundamental than these visible features. There are
many genes in plants which have equivalents in animals. Crucially,
for our topic, plants also have a highly developed epigenetic
system. They can modify histone proteins and DNA, just like animal
cells can, and in many cases use very similar epigenetic enzymes to
those used by animals, including humans.
These genetic and epigenetic similarities
all suggest that animals and plants have common ancestors. Because
of our common ancestry, we’ve inherited similar genetic and
epigenetic tool kits.
Of course, there are also really
important differences between plants and animals. Plants can create
their own food, but animals can’t do this. Plants take in basic
chemicals in the environment, especially water and carbon dioxide.
Using energy from sunlight, plants can convert these simple
chemicals into complex sugars such as glucose. Nearly all life on
planet earth is dependent directly or indirectly on this amazing
process of photosynthesis.
There are two other ways in which plants
and animals are very different. Most gardeners know that you can
take a cutting from a growing plant – maybe just a small shoot –
and create an entire new plant from this. There are very few
animals where this is possible, and certainly no advanced ones.
True, if certain species of lizard lose their tail, the animal can
grow a new one. But they can’t do this the other way around. We
can’t grow a new lizard from a discarded bit of tail.
This is because in most adult animals the
only genuinely pluripotent stem cells are the tightly controlled
cells of the germline which give rise to eggs or sperm. But active
pluripotent stem cells are a completely normal part of a plant. In
plants these pluripotent stem cells are found at the tips of stems
and the tips of roots. Under the right conditions, these stem cells
can keep dividing to allow the plant to grow. But under other
conditions, the stem cells will differentiate into specific cell
types, such as flowers. Once such a cell has become committed to
becoming part of a petal, for example, it can’t change back into a
stem cell. Even plant cells roll down Waddington’s epigenetic
landscape eventually.
The other difference between plants and
animals is really obvious. Plants can’t move. When environmental
conditions change, the plant must adapt or die. They can’t out-run
or out-fly unfavourable climates. Plants have to find a way of
responding to the environmental triggers all around them. They need
to make sure they survive long enough to reproduce at the right
time of year, when their offspring will have the greatest chance of
making it as new individuals.
Contrast this with a species such as the
European swallow (Hirundo rustica) which winters in South
Africa. As summer approaches and conditions become unbearable the
swallow sets off on an epic migration. It flies up through Africa
and Europe, to spend the summer in the UK where it raises its
young. Six months later, back it goes to South Africa.
Many of a plant’s responses to the
environment are linked to changes in cell fate. These include the
change from being a pluripotent stem cell to becoming part of a
terminally differentiated flower in order to allow sexual
reproduction. Epigenetic processes play important roles in both
these events, and interact with other pathways in plant cells to
maximise the chance of reproductive success.
Not all plants use exactly the same
epigenetic strategies. The best-characterised model system is an
insignificant looking little flowering plant called Arabidopsis
thaliana. It’s a member of the mustard family and looks like
any nondescript weed you can find on any patch of wasteland. Most
of the leaves grow close to the ground in a rosette shape. It
produces small white flowers on a stem about 20–25 centimetres
high. It’s been a useful model system for researchers because its
genome is very compact, which makes it easy to sequence in order to
identify the genes. There are also well-developed techniques for
genetically modifying Arabidopsis thaliana. This makes it
relatively straightforward for scientists to introduce mutations
into genes to investigate their function.
Arabidopsis thaliana seeds
typically germinate in early summer in the wild. The seedlings
grow, creating the rosette of leaves. This is called the vegetative
phase of plant growth. In order to produce offspring,
Arabidopsis thaliana generates flowers. It is structures in
the flowers that will generate the new eggs and sperm that will
eventually lead to new zygotes, which will be dispersed in
seeds.
But here’s the problem for the plant. If
it flowers late in the year, the seeds it produces will be wasted.
That’s because the weather conditions won’t be right for the new
seeds to germinate. Even if the seeds do manage to germinate, the
tender little seedlings are likely to be killed off by harsh
weather like frost.
The adult Arabidopsis thaliana
needs to keep its powder dry. It has a much greater chance of lots
of its offspring surviving if it waits until the next spring until
it flowers. The adult plant can survive winter weather that would
kill off a seedling. This is exactly what Arabidopsis
thaliana does. The plant ‘waits’ for spring and only then does
it produce flowers.
The rites of spring
The technical term for this is
vernalisation. Vernalisation means that a plant has to undergo a
prolonged cold period (winter, usually) before it can flower. This
is very common in plants with an annual life-cycle, especially in
the temperate regions of the earth where the seasons are
well-defined. Vernalisation doesn’t just affect broad-leaved plants
like Arabidopsis thaliana. Many cereals also show this
effect, especially crops like winter barley and winter wheat. In
many cases, the prolonged period of cold needs to be followed by an
increase in day length if flowering is to take place. The
combination of the two stimuli ensures that flowering occurs at the
most appropriate time of year.
Vernalisation has some very interesting
features. When the plant first begins to sense and respond to cold
weather, this may be many weeks or months before it starts to
flower. The plant may continue to grow vegetatively through cell
division during the cold period. When new seeds are produced, after
the vernalisation of the parent plant, the seeds are ‘reset’. The
new plants they produce from the seeds will themselves have to go
through their own cold season before flowering1.
These features of vernalisation are all
very reminiscent of epigenetic phenomena in animals.
Specifically:
1. The plant displays some form
of molecular memory, because the stimulus and the final event are
separated by weeks or months. We can compare this with abnormal
stress responses in adult rodents that were ‘neglected’ as
infants.
2. The memory is maintained
even after cells divide. We can compare this with animal cells that
continue to perform in a certain way after a stimulus to the parent
cell, such as in normal development or in cancer
progression.
3. The memory is lost in the
next generation (the seeds). This is comparable with the way that
most changes to the somatic tissues are ‘wiped clean’ in animals so
that Lamarckian inheritance is exceptional, rather than
common.
So, at a phenomenon level,
vernalisation looks very epigenetic. In recent years, a number of
labs have confirmed that epigenetic processes underlie this, at the
chromatin modification level.
The key gene involved in vernalisation is
called FLOWERING LOCUS C or FLC for short. FLC
encodes a protein called a transcriptional repressor. It binds to
other genes and stops them getting switched on. There are three
genes that are particularly important for flowering in
Arabidopsis thaliana, called FT, SOC1 and
FD. Figure 15.1 shows how FLC
interacts with these genes, and the consequences this has for
flowering. It also shows how the epigenetic status of FLC changes
after a period of prolonged cold.
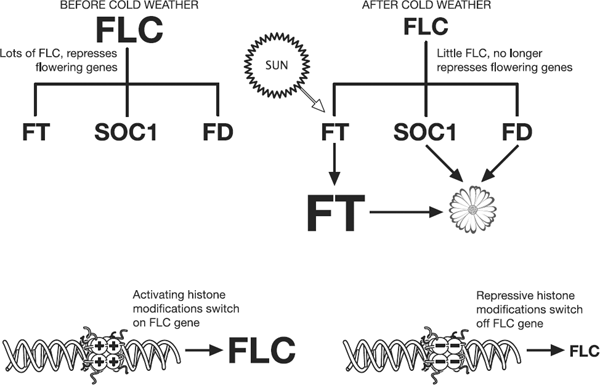
Figure 15.1
Epigenetic modifications regulate the expression of the FLC
gene, which represses the genes which promote flowering. The
epigenetic modifications on the FLC gene are controlled by
temperature.
Before winter, the FLC gene
promoter carries lots of histone modifications that switch on gene
expression. Because of this, the FLC gene is highly
expressed, and the protein it codes for binds to the target genes
and represses them. This keeps the plant in its normal growing
vegetative phase. After winter, the histone modifications at the
FLC gene promoter change to repressive ones. These switch
off the FLC gene. The FLC protein levels drop, which removes
the repression on the target genes. The increased periods of
sunlight during spring activate expression of the FT gene.
It’s essential that FLC levels have gone down by this stage,
because if FLC levels are high, the FT gene finds it
difficult to react to the stimulus from sunlight2.
Experiments with mutated versions of
epigenetic enzymes have shown that the changes in histone
modifications at the FLC gene are critically important in
controlling the flowering response. For example, there is a gene
called SDG27 which adds methyl groups to the lysine amino
acid at position 4 on histone H3, so it is an epigenetic writer.
This methylation is associated with active gene expression. The
SDG27 gene can be mutated experimentally, so that it no
longer encodes an active protein. Plants with this mutation have
less of this active histone modification at the FLC gene
promoter. They produce less FLC protein, and so aren’t so good at
repressing the genes that trigger flowering. The SDG27
mutants flower earlier than the normal plants3. This
demonstrates that the epigenetic modifications at the FLC
promoter don’t simply reflect the activity levels of the gene, they
actually alter the expression. The modifications do actually cause
the change in expression.
Cold weather induces a protein in plant
cells called VIN3. This protein can bind to the FLC
promoter. VIN3 is a type of protein called a chromatin remodeller.
It can change how tightly chromatin is wound up. When VIN3 binds to
the FLC promoter, it alters the local structure of the
chromatin, making it more accessible to other proteins. Often,
opening up chromatin leads to an increase in gene expression.
However, in this case, VIN3 attracts yet another enzyme that can
add methyl groups to histone proteins. However, this particular
enzyme adds methyl groups to the lysine amino acid at position 27
on histone H3. This modification represses gene expression and is
one of the most important methods that the plant cell uses to
switch off the FLC gene4,5.
This still raises the question of how
cold weather results in epigenetic changes to the FLC gene
specifically. What is the targeting mechanism? We still don’t know
all the details, but one of the stages has been elucidated.
Following cold weather, the cells in Arabidopsis thaliana
produce a long RNA, which doesn’t code for protein. This RNA is
called COLDAIR. The COLDAIR non-coding RNA is localised
specifically at the FLC gene. When localised, it binds to
the enzyme complex that creates the important repressive mark at
position 27 on histone H3. COLDAIR therefore acts as a targeting
mechanism for the enzyme complex6.
When Arabidopsis thaliana produces
new seeds, the repressive histone marks at the FLC gene are
removed. They are replaced by activating chromatin modifications.
This ensures that when the seeds germinate the FLC gene will
be switched on, and repress flowering until the new plants have
grown through winter.
From these data we can see that flowering
plants clearly use some of the same epigenetic machinery as many
animal cells. These include modifications of histone proteins, and
the use of long non-coding RNAs to target these modifications.
True, animal and plant cells use these tools for different
end-points – remember the orthopaedic surgeon and the carpenter
from the previous chapter – but this is strong evidence for common
ancestry and one basic set of tools.
The epigenetic similarities between
plants and animals don’t end here either. Just like animals, plants
also produce thousands of different small RNA molecules. These
don’t code for proteins, instead they silence genes. It was
scientists working with plants who first realised that these very
small RNA molecules can move from one cell to another, silencing
gene expression as they go7,8. This spreads the
epigenetic response to a stimulus from one initial location to
distant parts of the organism.
The kamikaze cereal
Research in Arabidopsis thaliana
has shown that plants use epigenetic modifications to regulate
thousands of genes9. This regulation probably
serves the same purposes as in animal cells. It helps cells to
maintain appropriate but short-term responses to environmental
stimuli, and it also locks differentiated cells in permanent
patterns of specific gene expression. Because of epigenetic
mechanisms we humans don’t have teeth in our eyeballs, and plants
don’t have leaves growing out of their roots.
Flowering plants share a characteristic
epigenetic phenomenon with mammals, and with no other members of
the animal kingdom. Flowering plants are the only organisms we know
of besides placental mammals in which genes are imprinted.
Imprinting is the process we examined in Chapter 8, where the expression pattern of a gene
is dependent on whether it was inherited from the mother or
father.
At first glance, this similarity between
flowering plants and mammals seems positively bizarre. But there’s
an interesting parallel between us and our floral relations. In all
higher mammals, the fertilised zygote is the source of both the
embryo and the placenta. The placenta nourishes the developing
embryo, but doesn’t ultimately form part of the new individual.
Something rather similar happens when fertilisation occurs in
flowering plants. The process is slightly more complicated, but the
final fertilised seed contains the embryo and an accessory tissue
called the endosperm, shown in Figure
15.2.
Just like the placenta in mammalian
development, the endosperm nourishes the embryo. It promotes
development and germination but it doesn’t contribute genetically
to the next generation. The presence of any accessory tissues
during development, be this a placenta or an endosperm, seems to
favour the generation of imprinted control of the expression of a
select group of genes.
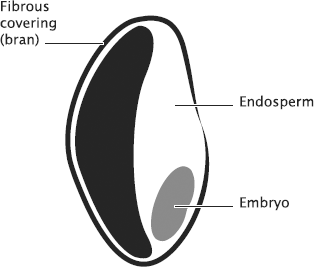
Figure 15.2 The
major anatomical components of a seed. The relatively small embryo
that will give rise to the new plant is nourished by the endosperm,
in a manner somewhat analogous to the nourishment of mammalian
embryos by the placenta.
In fact, something very sophisticated
happens in the endosperm of seeds. Just like most animal genomes,
the genomes of flowering plants contain retrotransposons. These are
usually referred to as TEs – transposable elements. These are the
repetitive elements that don’t encode proteins, but can cause havoc
if they are activated. This is especially because they can move
around in the genome and disrupt gene expression.
Normally such TEs are tightly repressed,
but in the endosperm these sequences are switched on. The cells of
the endosperm create small RNA molecules from these TEs. These
small RNAs travel out from the endosperm into the embryo. They find
the TEs in the embryo’s genome that have the same sequence as
themselves. These TE small RNA molecules then seem to recruit the
machinery that permanently inactivates these potentially dangerous
genomic elements. The risk to the endosperm genome through
reactivation of the TEs is high. But because the endosperm doesn’t
contribute to the next generation genetically, it can undertake
this suicide mission, for the greater good10,11,12,13.
Although mammals and flowering plants
both carry out imprinting, they seem to use slightly different
mechanisms. Mammals inactivate the appropriate copy of the
imprinted gene by using DNA methylation. In plants, the
paternally-derived copy of the gene is always the one that carries
the DNA methylation. However, it’s not always this methylated copy
of the gene that is inactivated14. In plant imprinting,
therefore, DNA methylation tells the cell how a gene was inherited,
not how the gene should be expressed.
There are some fundamental aspects of DNA
methylation that are quite similar between plants and animals.
Plant genomes encode active DNA methyltransferase enzymes, and also
proteins that can ‘read’ methylated DNA. Just like primordial germ
cells in mammals, certain plant cells can actively remove
methylation from DNA. In plants, we even know which enzymes carry
out this reaction15. One is called DEMETER,
after the mother of Persephone in Greek myths. Demeter was the
goddess of the harvest and it was because of the deal that she
struck with Hades, the god of the Underworld, that we have
seasons.
But DNA methylation is also an aspect of
epigenetics where there are clear differences in the way plants and
higher animals use the same basic system. One of the most obvious
differences is that plants don’t just methylate at CpG motifs
(cytosine followed by a guanine). Although this is the most common
sequence targeted by their DNA methyltransferases, plants will also
methylate a cytosine followed by almost any other base16.
A lot of DNA methylation in plants is
focused around non-expressed repetitive elements, just like in
mammals. But a big difference becomes apparent when we examine the
pattern of DNA methylation in expressed genes. About 5 per cent of
expressed plant genes have detectable DNA methylation at their
promoters, but over 30 per cent are methylated in the regions that
encode amino acids, in the so-called body of the genes. Genes with
methylation in the body regions tend to be expressed in a wide
range of tissues, and are expressed at moderate to high levels in
these tissues17.
The high levels of DNA methylation at
repetitive elements in plants are very similar to the pattern at
repetitive elements in the chromatin of higher animals such as
mammals. By contrast, the methylation in the bodies of widely
expressed genes is much more like that seen in honeybees (which
don’t methylate their repetitive elements). This doesn’t mean that
plants are some strange epigenetic hybrid of insects and mammals.
Instead, it suggests that evolution has a limited set of raw
materials, but isn’t too obsessive about how it uses
them.